Trial Finder Web App
Why: The Trial Finder Web Application is a web-based tool designed to help clinicians and care teams quickly access trial-specific data, enabling faster response times and improved trial matching for patients.
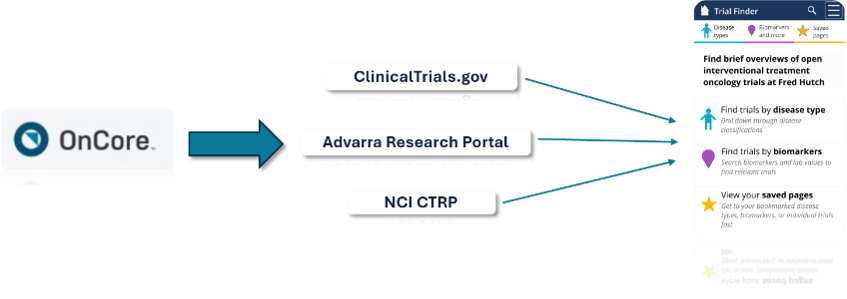
How: The app integrates data from OnCore CTMS, ClinicalTrials.gov, and the National Cancer Institute (NCI) to populate key trial information.
Data Included: Protocol metadata, staff contact details, trial attributes, biomarkers, and more.
Access: Visit https://trialfinder.seattlectms.org and log in using your UW NetID or Fred Hutch credentials.
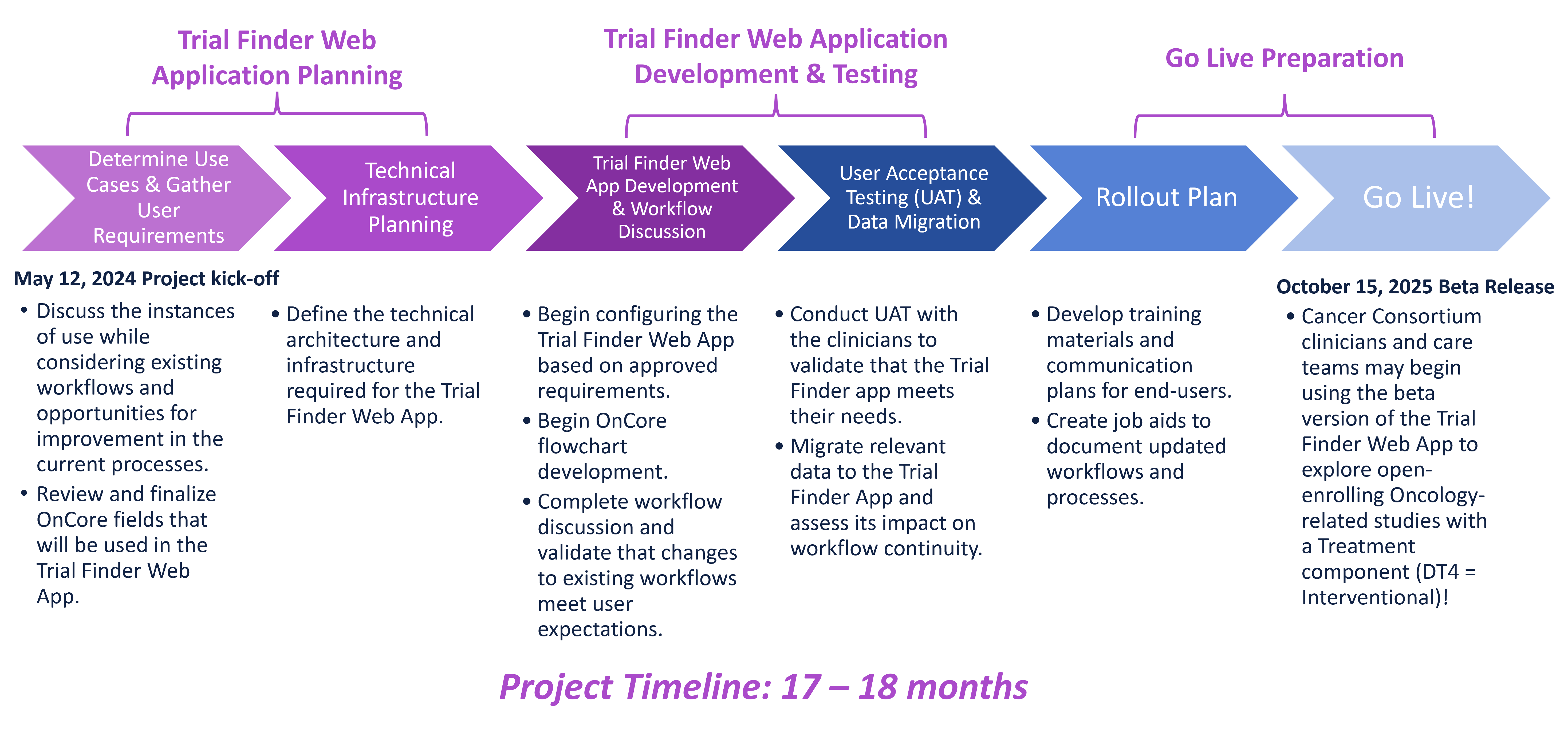
Production Release Plan & Timeline
Release Date: December 10, 2025
Scope: Open-enrolling interventional oncology treatment studies with fully completed data in OnCore.
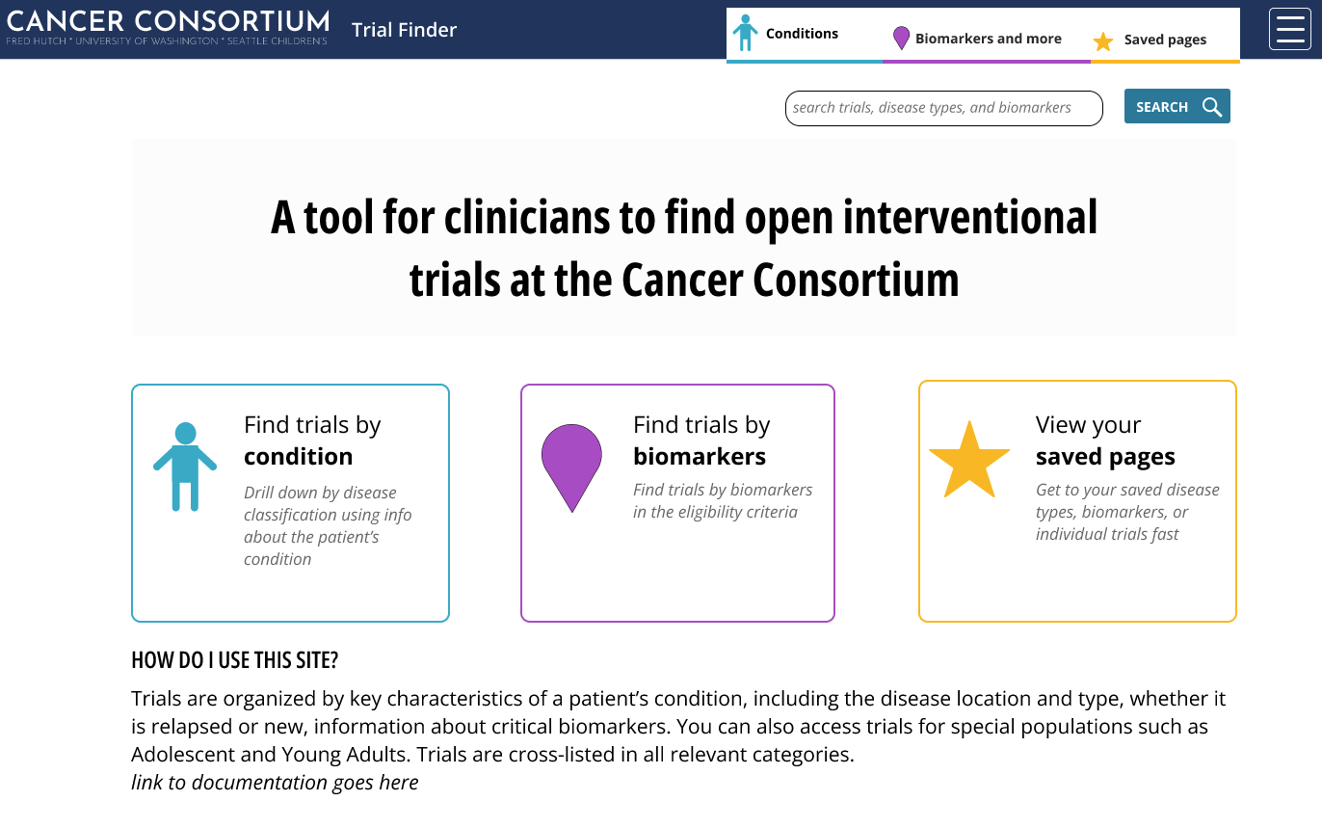
Clinicians and research staff can access the Trial Finder Web Application (login with UW NetID or FH credentials required) to search and explore open-enrolling studies for their patients.
Click on the tabs below to learn more about the different information available to study teams on the Trial Finder web app.
Trial Finder App – Frequently Asked Questions (FAQs)
General Information
What is Trial Finder?
Trial Finder is a web-based application designed to streamline patient recruitment for clinical trials. It provides clinicians and care teams with quick access to trial-specific data, helping accelerate the process of identifying and matching patients to appropriate trials.
Who can use Trial Finder?
Trial Finder is intended for clinical and research staff, including clinicians/investigators, advanced practice providers (APPs), clinical research nurses (CRNs), study coordinators, and recruitment specialists involved in matching patients to actively enrolling oncology trials.
What studies are displayed in the Trial Finder app?
The initial release includes actively enrolling oncology-related studies with a treatment component and an Interventional Data Table 4 report type. Study teams are encouraged to enter required data in OnCore during study setup, ideally post-SIV or at OTA, and no later than when the first patient is consented.
Is there a cost to use the application?
No. Trial Finder is free for staff affiliated with the Cancer Consortium. Login requires a University of Washington (UW) NetID or Fred Hutch credential.
How does Trial Finder help with patient recruitment?
The tool enables users to search trials by eligibility criteria, view real-time recruitment status, and connect with research teams, reducing manual effort and improving matching accuracy.
Functionality & Features
What are the key features of the Trial Finder app?
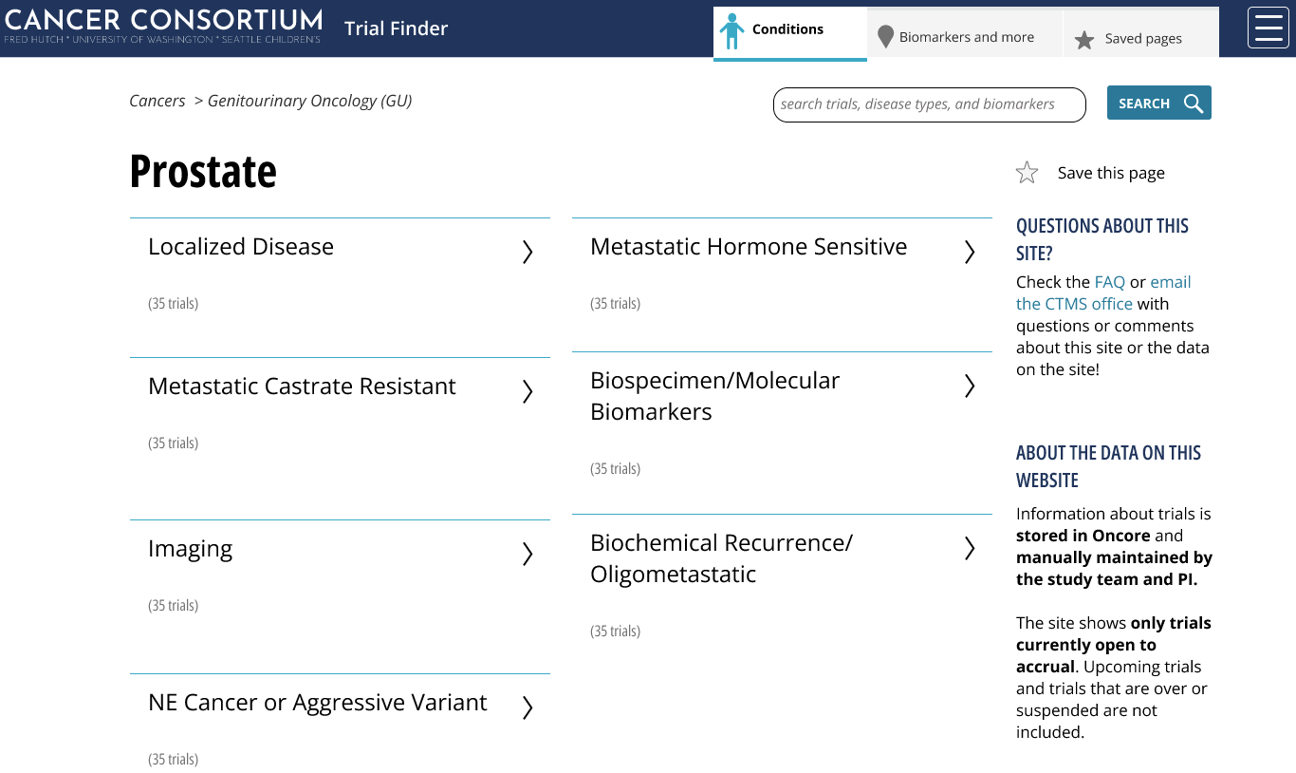
Trial Finder offers multiple navigation paths, including condition-based and biomarker-based filtering. Users can refine searches by study phase, participant age, investigator, and more. The responsive design ensures consistent performance across desktop and mobile devices, and cached data improves reliability during connectivity issues.
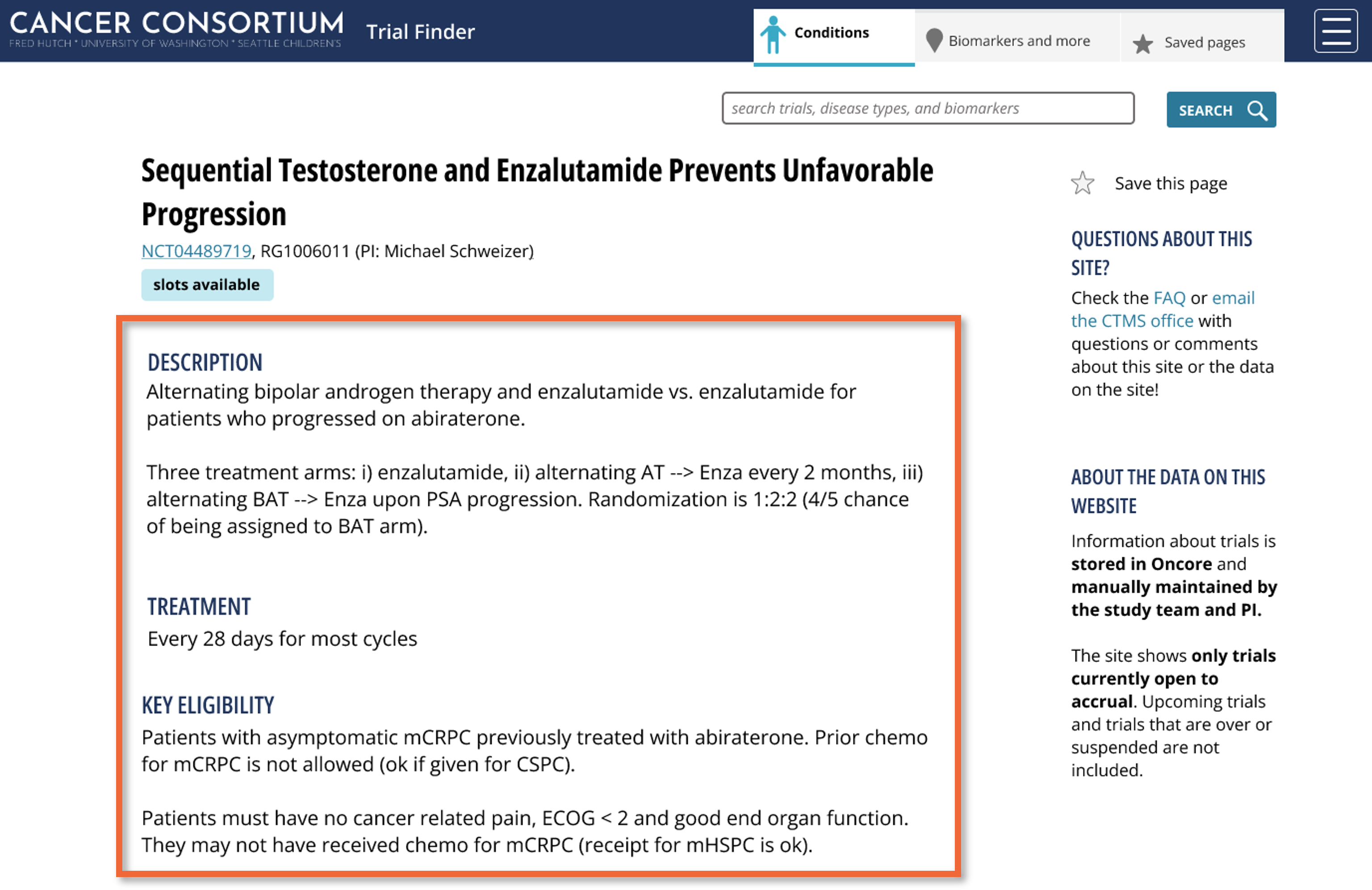
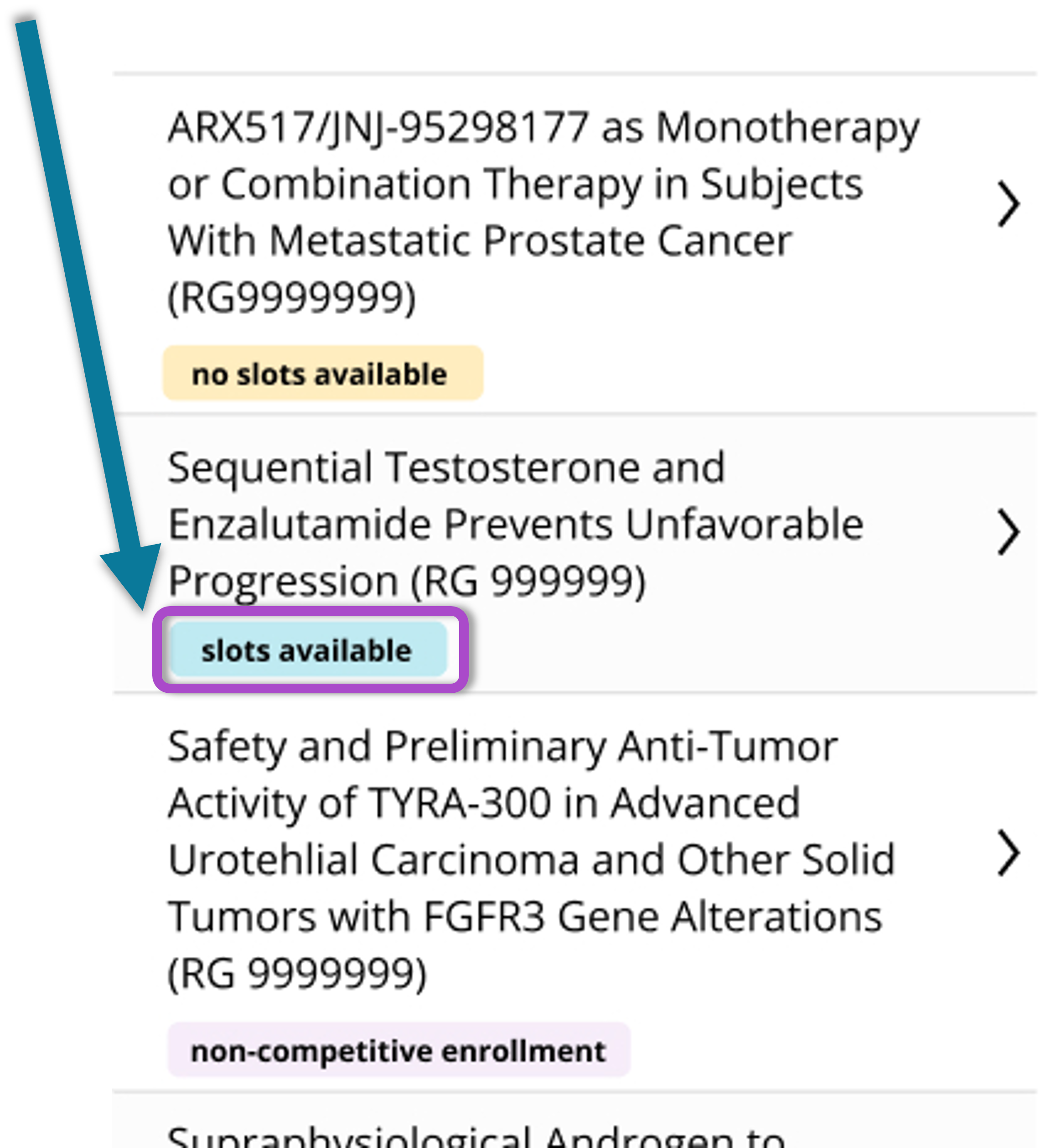
The Key Trial Information page provides a concise overview of each trial. including a brief study description, abbreviated treatment schedule, and key eligibility criteria, allowing clinicians to quickly assess trial suitability without reviewing the full protocol.
Clear eligibility criteria reduce missed opportunities and promote collaboration by making trial details more visible. Color-coded, pill-style indicators display slot availability, a key factor in guiding enrollment decisions. Real-time slot data improves matching accuracy and streamlines recruitment.
How do I search trials for eligible patients?
Use the advanced search feature to filter trials by condition, research group, biomarkers, participant age, study phase, investigator, and other criteria. Keyword searches and saved queries are also supported.
Can I search for trials by investigator or site location?
Yes. The advanced search feature allows filtering by investigator and site-specific attributes.
Can I save search results?
Yes. Users can bookmark conditions, biomarkers, and individual trials, which are accessible under the Saved Pages section.
Will future releases include observational or diagnostic trials?
The initial release focuses on interventional oncology treatment trials. Expansion to include observational or diagnostic trials is under consideration for future iterations.
Trial Data Management
How often is the trial data updated?
Trial data is refreshed daily via an overnight data transfer. Only studies with an Open to Accrual (OTA) status in OnCore are displayed.
How often should study teams update OnCore data?
Study teams should update OnCore as needed to reflect current trial information. Updates are required for protocol amendments affecting trial description, treatment schedule, eligibility criteria, slot availability, or recruitment contacts.
How often is slot availability updated?
Slot availability is refreshed daily during the overnight data transfer. However, the data will only reflect changes if the slot status has been updated by the study team. Study teams are responsible for updating slot availability in OnCore whenever there is a change and must record a ‘Last Updated’ date to indicate when the information was last modified.
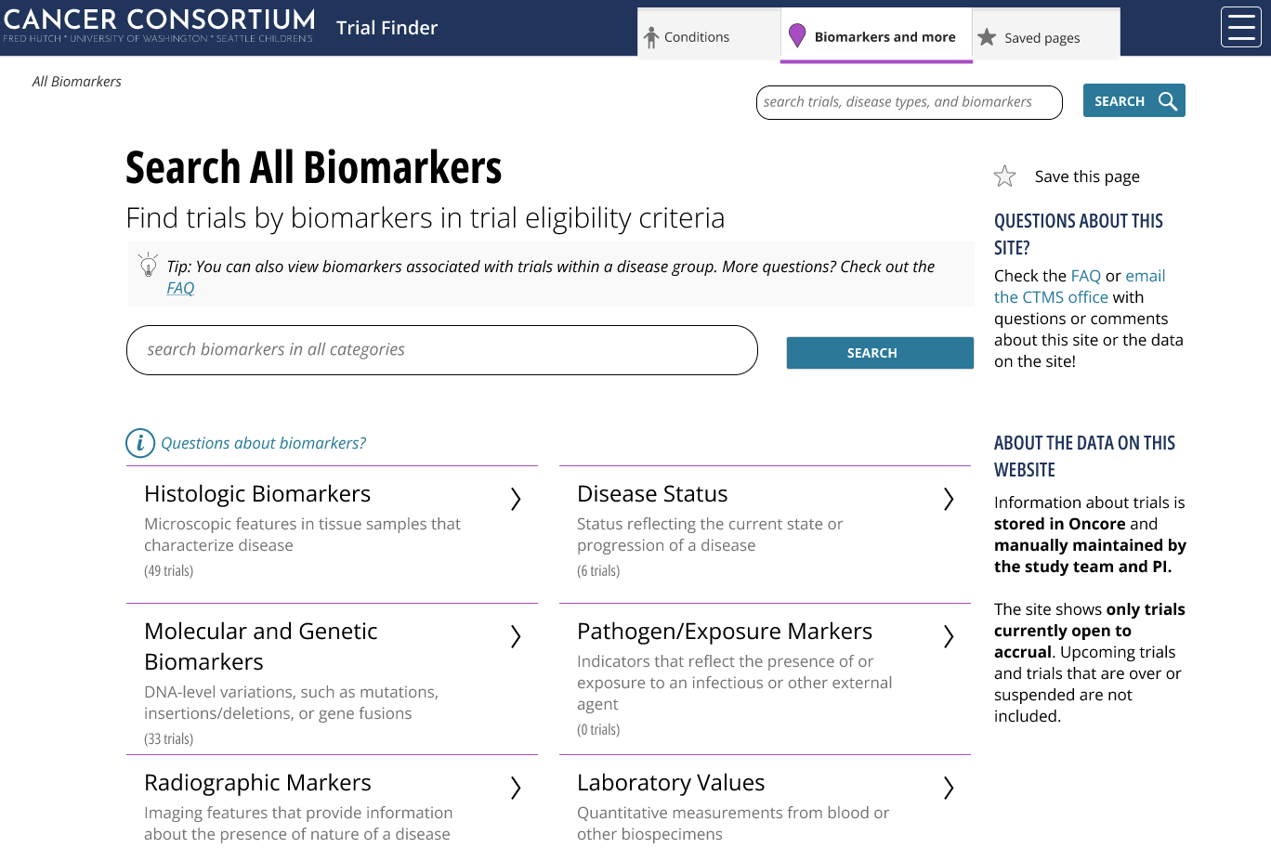
What type of biomarkers are displayed?
The initial release displays biomarkers from inclusion criteria, organized into nine categories, from genetic markers to laboratory values and disease statuses. This enhances searchability and supports efficient patient-trial matching.
Should exclusion biomarkers be included?
Yes. Study teams should list exclusion biomarkers in the Key Eligibility section of the Key Trial Information page to provide valuable context for clinicians.
Do biomarkers apply to all disease types in a study?
Not necessarily. Biomarkers are designated at the protocol level and may apply only to specific disease types. For example, ‘NECTIN4 Positive’ may be relevant for cervical cancer but not urothelial cancer within the same trial.
User Access & Permissions
How do I access the Trial Finder web application?
Visit https://trialfinder.seattlectms.org and log in using your UW NetID or Fred Hutch credentials.
How do I create an account?
No account creation is required. Trial Finder is available to all Cancer Consortium members via UW NetID or Fred Hutch login.
Is Trial Finder available to the public or external institutions?
No. Trial Finder is an internal tool. We are exploring guest login options for external providers and updating the Fred Hutch public website to display trial information externally.
Data Privacy & Security
Where is the data coming from?
Trial Finder pulls data from OnCore CTMS, ClinicalTrials.gov, and the NCI to display protocol metadata, staff contacts, trial attributes, biomarker data, and more.
Is patient data protected?
Yes. Trial Finder does not store or display identifiable patient data.
Will usage data be tracked or reported?
Usage metrics will be collected to improve the application and optimize functionality. No identifiable user data is shared.
Support & Troubleshooting
Who do I contact for support or provide feedback?
Use the REDCap survey link at the bottom of the Trial Finder homepage or email CTMS@fredhutch.org.
Is there a user guide or training available?
Yes. Resources are available on the CTMS Documents & Videos webpage (OnCore login required), including job aids, work instructions, and a recorded webinar demo.
How do I report inaccurate or outdated trial information?
Users can report issues to CTMS@fredhutch.org. The CTMS team also conducts proactive data quality checks.