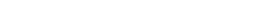24 Jul Using REDCap to consent research participants
PLEASE NOTE: The information on this page is out of date. A new post with the latest information about the REDCap e-Consent platform is coming soon. In the meantime, please contact redcaphelp@uw.edu with any questions.
Obtaining written consent is a critical step in the clinical research process, but managing paper forms can be a major hassle. REDCap offers a digital method to acquire and store participant consent through a new e-Consent Framework and PDF Auto-Archiver.
What is e-Consent?
Electronic-Consent (e-Consent) is a platform for consenting research participants using a computer-based consent form rather than traditional paper documentation. REDCap now has a feature that implements consent forms through an online survey which can be accessed on a computer, mobile phone, or tablet.
Participants can ‘sign’ their consent by typing in their name or by utilizing REDCap’s ‘Signature’ field type on the survey. Please note that the signature process is NOT implemented by REDCap automatically; a survey must be constructed using one of these two methods in order to capture a signature.
How the e-Consent Framework works
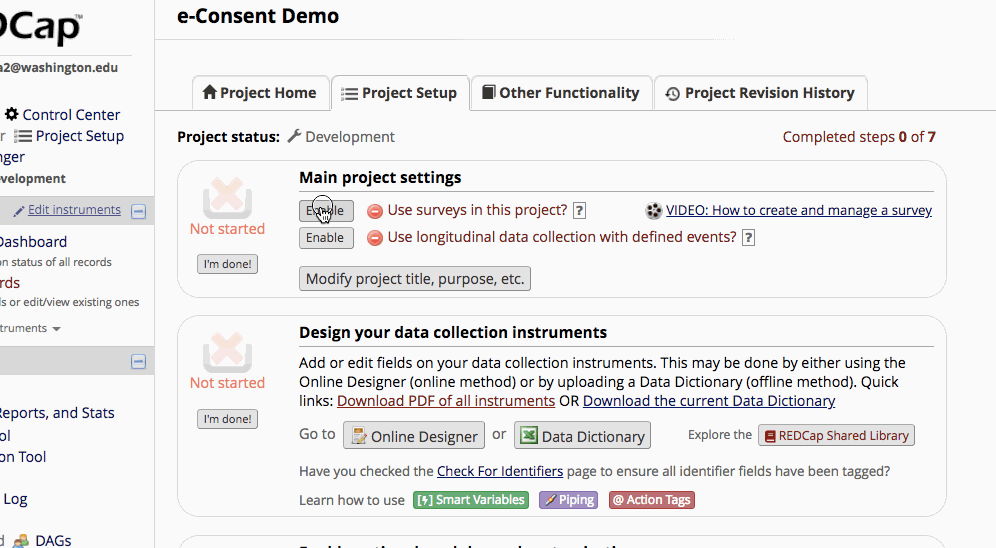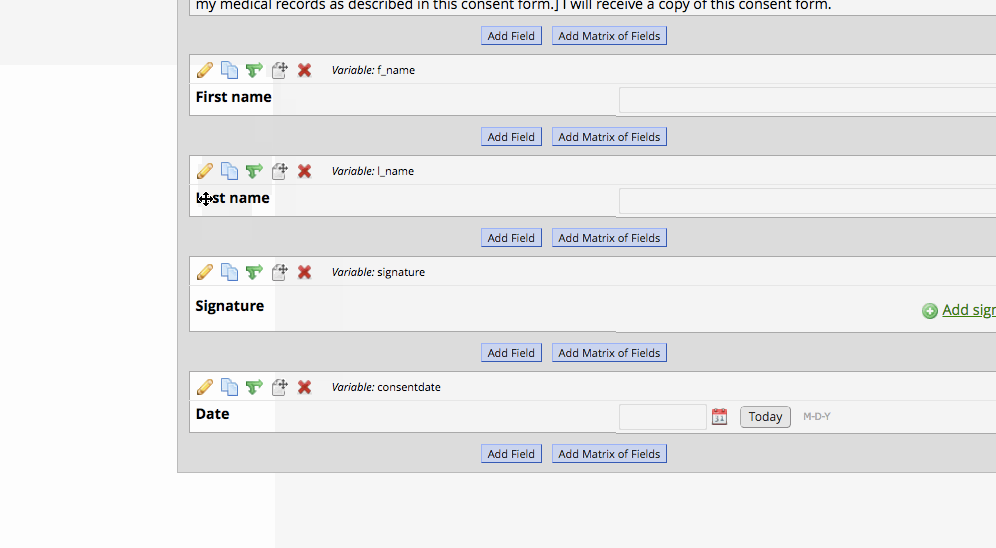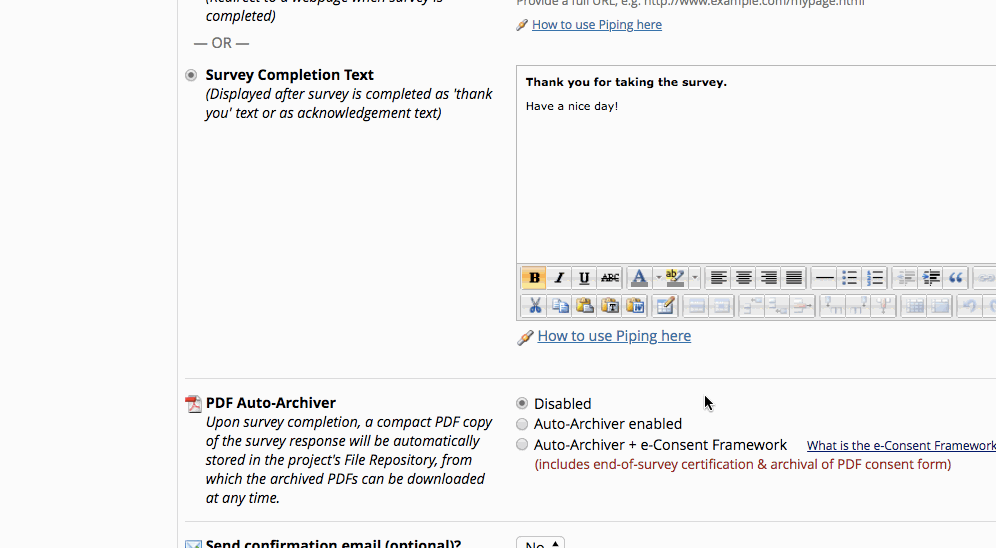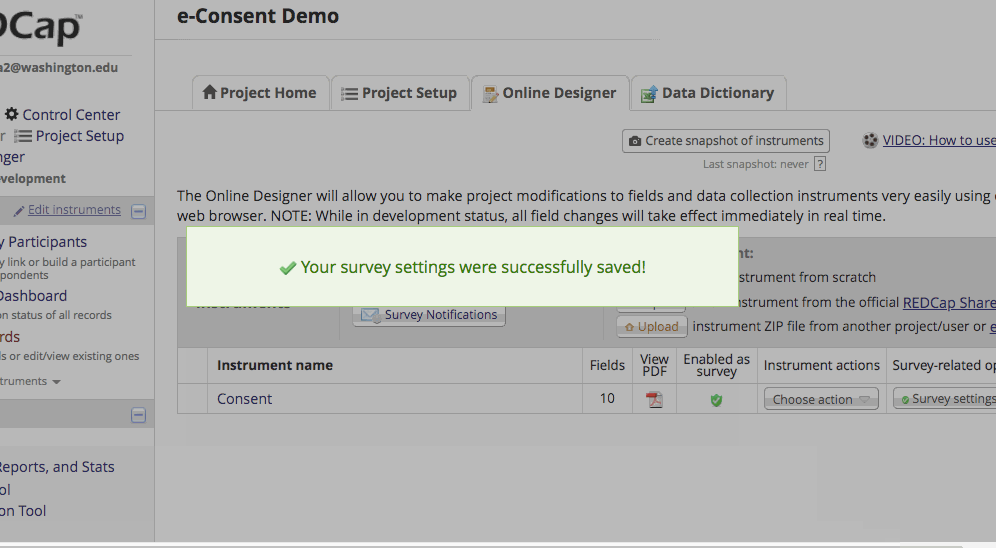
The REDCap e-Consent Framework provides standardized tools to obtain consent and store consent documentation with a certification screen and a storage function which automatically generates a ‘hard-copy’ PDF of the signed form.
The ‘Auto-Archiver + e-Consent Framework’ survey option adds two things to the typical survey-taking process.
- Before a participant completes the survey, an extra certification page is added to the end of the survey that displays an in-line PDF copy of the document in which they will be asked to confirm that all information in the document is correct.
- The survey will not be considered complete until they fulfill the certification step.
- Upon completion of the survey, a static copy of their responses in the form of a consent-specific PDF will be stored in the project’s File Repository.
- The consent-specific PDF will have the values of the e-Consent Framework Options inserted at the bottom of each page in the PDF.
- These values (i.e., name, date of birth, etc.) are added to the PDF as extra documentation of the identity of the person who is consenting.
e-Consent at the University of Washington
As of October 15, 2020, the University of Washington Human Subjects Division has approved the use of the REDCap e-consent framework to obtain and store legally valid electronic consent signatures. This is the result of a collaboration between the Human Subjects Division, ITHS, the UW Attorney General’s office, UW Privacy, UW Medicine, UW School of Medicine, and regional HIPAA Privacy officials. Please review the announcement by UW Human Subjects Division for more information.
Part of the collaboration between the ITHS REDCap administration team and UW Human Subjects Division was to set up a process for consenting participants in REDCap including guidelines and templates to use when using the e-consent framework. On October 28, 2020, Ashleigh Lewis, Lead REDCap Administrator; Bas de Veer, Biomedical Informatics Service Manager; and Jason Malone, Assistant Director, Regulatory Affairs, conducted a training for researchers going over the process of e-consent in REDCap. In accordance with the training we have built a how-to guide for adding the e-consent template in the ITHS REDCap instance as well as incorporating the template into an already built project. This guide also contains an embedded video of the live training that occurred in October. This guide will stay up to date with any template changes.
Please note: The templates will not stay up to date with consent language; the templates will always need adjustment with your study’s approved consent language. You should always go directly to the UW Human Subjects Division website for the current consent language template.
Setting up e-consent
- Enable surveys on the “Project Setup” page.
- Set up a survey instrument with the consent language as well as first, last, signature, and a date validated field. Optional fields may be included, such as ‘date of birth’.
- Enable the consent instrument as a survey.
- If it is already enabled go into survey settings and turn on “auto-archiver + e-Consent Framework”.
- A pop up will appear requesting first and last name variables as well as version number. *Versioning of a form is a concept whereby you may give it a number or alpha-numeric designation to represent the current version.
- If the form is modified AFTER data collection begins, then it is recommended that a new version be applied. For example, the first version might simply be ‘1’, and after collecting the consent of a few participants, a question is modified or added, which represents a new version of the form, so you might increment the version to ‘2’ (and so forth).
- You can select the optional fields like date of birth or the e-consent type.
*The e-Consent ‘type’ is another free-form text field that can be used to signify the type of e-Consent that this survey represents (e.g., pediatric). ‘Type’ is often used to distinguish between multiple e-Consent forms within a project. - Finish up by clicking the “Save Changes” button.
*Consent version and type are both free-form text fields whose value will be inserted at the footer of each page in the PDF.
Setting up the Project
How participants use e-Consent
The participant will open the survey and read through the consent form. When they get to the bottom, they will have the opportunity to fill in their information and sign their name if they agree to participate. They will select “Next Page” and a read only copy of the consent will be generated that they can review, download, and/or print. At the bottom of the page they will need to select “I certify that all the information in the document above is correct, and I understand that signing this form electronically is the equivalent of signing a physical document.” Once this is selected they will be able to submit the survey.
A closer look at the process

For more information, visit the e-Consent demo project to see an example of how this would work for the participant.
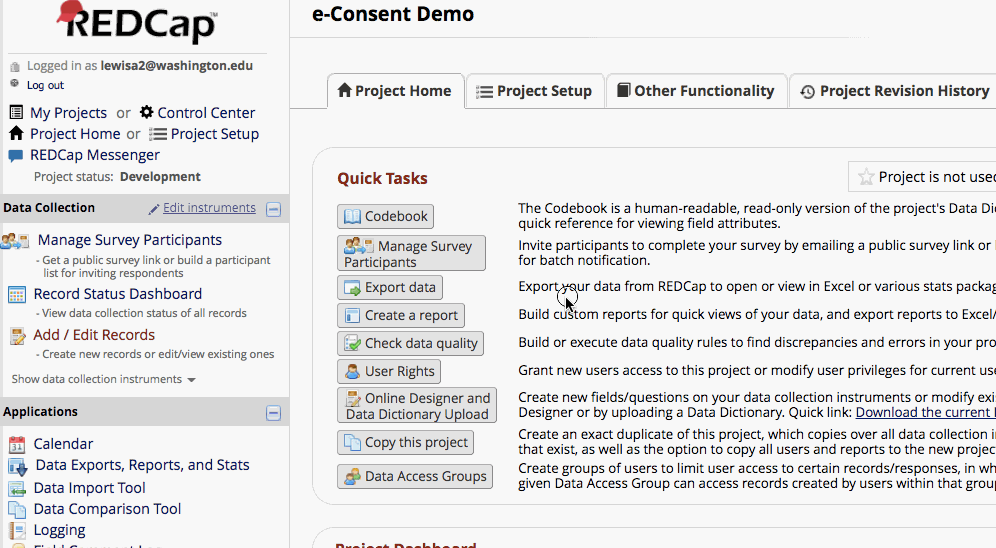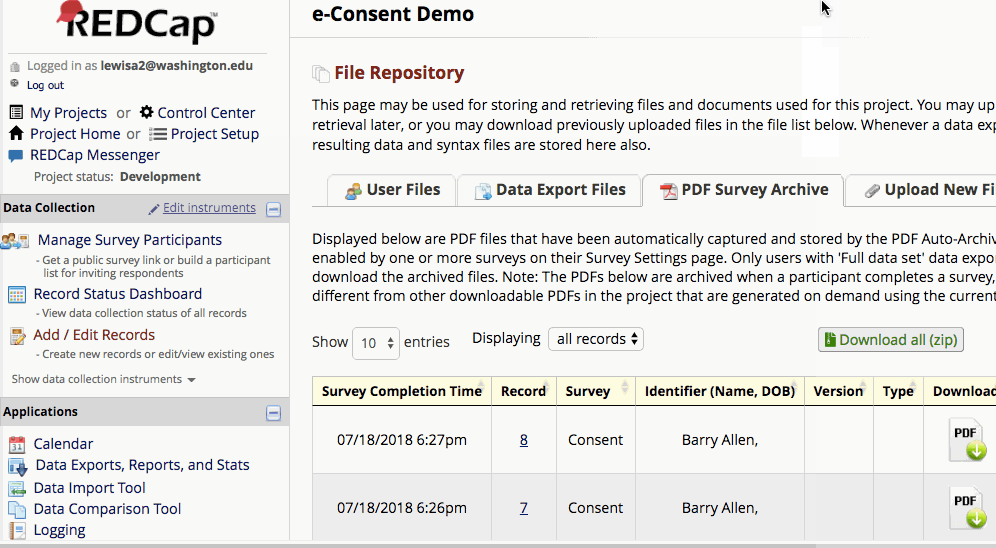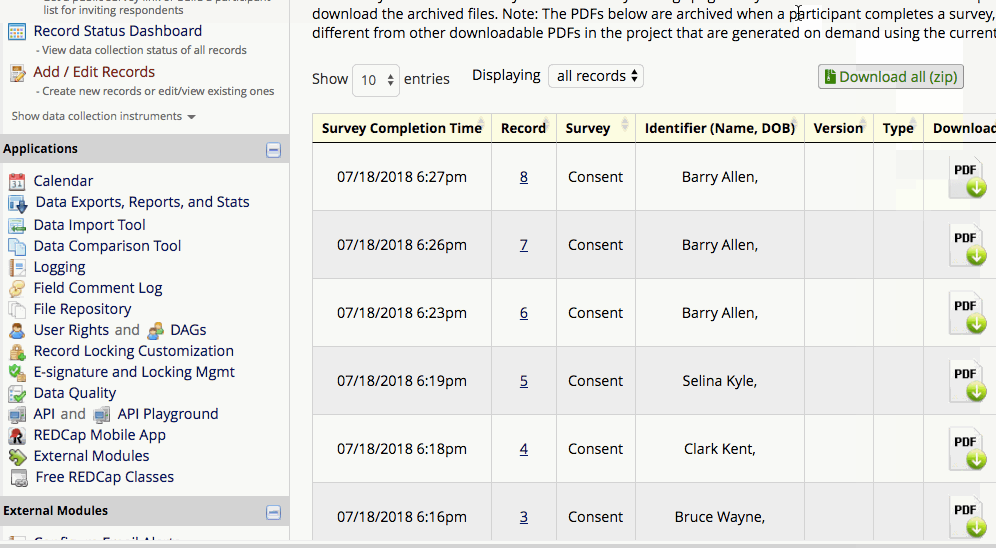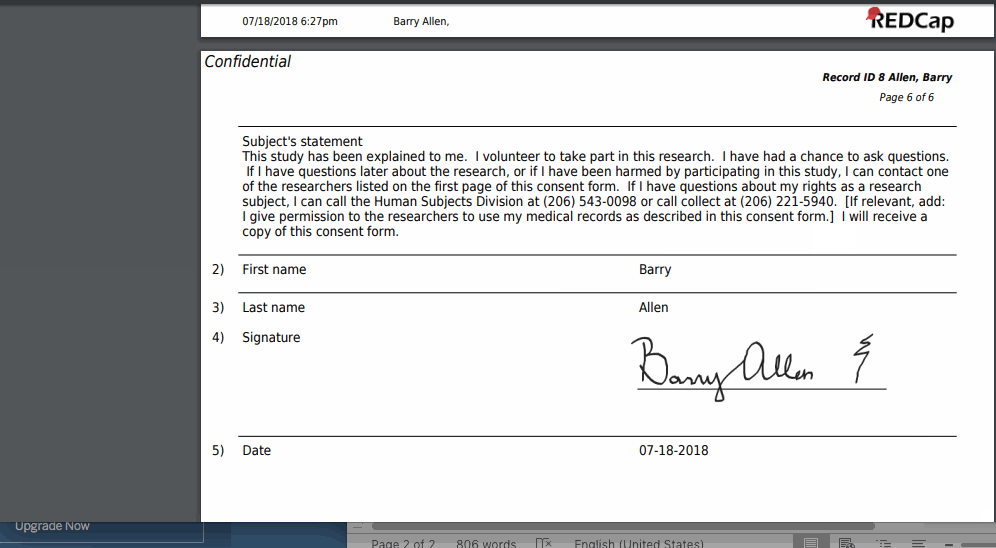
Where are completed PDFs stored?
The completed PDFs are in the File Repository under “PDF Survey Archive.” Files can be downloaded as individual records or bundles in a ZIP file. Note: only users with ‘Full data set’ data export privileges will be able to download the archived files. The e-Consent Framework also records the IP address of the participant and displays this information in the file repository in order to help regulate potential duplicate forms from a single IP address.
How to view/download completed PDFs